hidden
Over 10 years experience of Traceability Solutions
By Pharmatrax Author
Category: Technoloy
 No Comments
No Comments
3 EPCIS Data Exchange Issues in Pharma Traceability
Conformance testing services have emerged to cut down on wasted back-and-forth between manufacturers and trading partners to get efficient data exchange up and running.
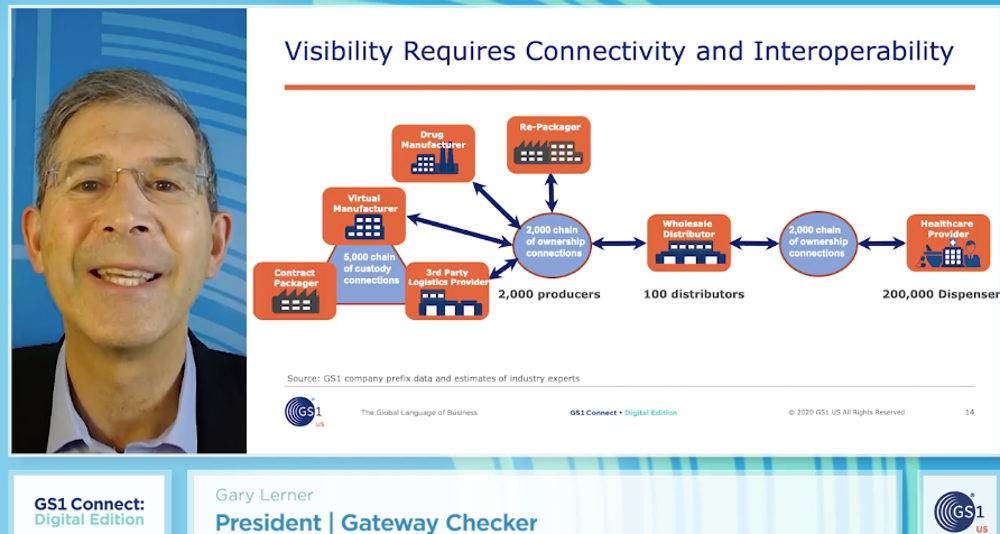
Pharma companies continue to make inroads to meet the DSCSA 2023 requirement for unit-level traceability at each change of ownership between trading partners. “Capturing and exchanging information at this level of specificity requires a common information framework,” explained Gary Lerner, president at Gateway Checker Corporation, at the GS1 Connect: Digital Edition (virtual) conference this week.
“This information framework captures what happens in the physical world with an electronic twin, essentially an information encoded representation of packing and shipping events that can be exchanged with trading partners. What’s needed to accomplish that is a common vocabulary and structure that enables trading partners to then share and exchange essential trading information in a consistent, accurate and interoperable manner,” said Lerner. Without a common standardized approach, there will be considerable waste in time and effort with trading partners negotiating the structure and content in each point of connection.
EPCIS
Lerner pointed to the value of the global GS1 standard EPCIS (Electronic Product Code Information Services), which enables the common information exchange language that can be shared. “However, EPCIS is a general purpose exchange standard. Application-specific standards are therefore necessary to tailor EPCIS to suit a specific industry and application. In 2015, the GS1 US Healthcare working group recruited more than 50 companies representing a cross section of the pharmaceutical industry to adapt EPCIS to address DSCSA specific requirements. And in 2016, the application standards governing DSCSA traceability were published. Adopting these standards therefore helps different businesses within the drug supply chain to more efficiently and effectively connect and share information,” he said.
Challenges of data exchange
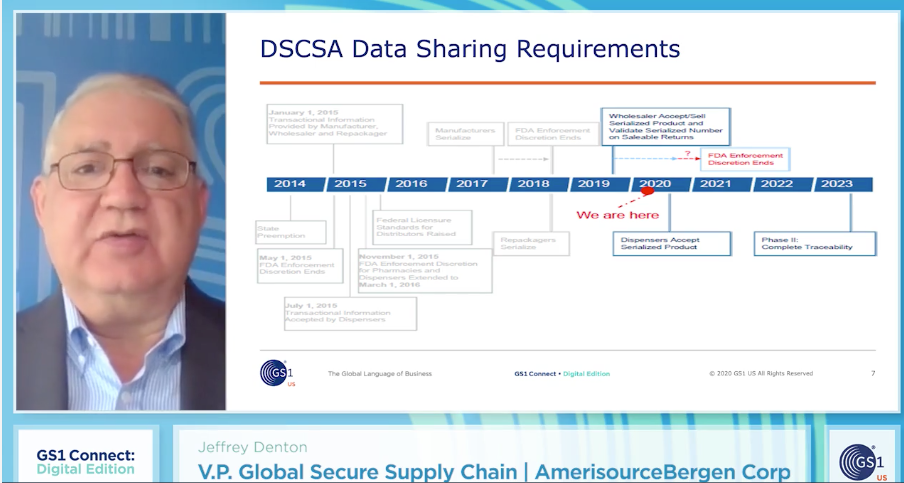
As testing is underway, AmerisourceBergen Corporation (ABC) is already seeing issues with EPCIS files coming in from drug manufacturers. Jeff Denton, vice president, global secure supply chain at ABC, said at this point, they’ve received about 30 to 40 files and “nearly every single one of them had issues upfront with their initial testing. I don’t think I had a single file come in for the first time that was accurate.”
He shared three of the issues observed:
Event time is missing: Denton explains that event time describes when something occurs such as commissioning a bottle on the packaging line or packing it into a case or shipping it. Those events need to be in a sequence. When the data arrives at your downstream trading partner, he said, “The very first thing their system is looking for is making sure it’s all in sequence, and they use that event time as a process to do that.”
In one case, the majority of the file was fine, but some events had dates missing. For some reason, there isn’t consistency in the event time being represented accurately or being present.
Duplicate SGTINs (serialized GTINs): One example he referred to is a case of 12 identical bottles. “They all have the same GTIN, but the serial numbers on each bottle are unique. We’re getting data that now is showing the exact same serial number on more than one bottle, which shouldn’t happen, whether it was in one case or different cases, but we are finding that,” Denton said.
No children: Another major error they’ve observed is a file lacking child records. “We get the serial number for the case, but we don’t get the serial number for the contents of that case,” he explained.
He recalled an example for a large file, where they had the children and the case for the majority of the shipment, but only had the case for some products. “This is not [a product] that would be going through downstream distribution at a case level. That would be understandable. This is something that is shipped typically at the unit level.
“At ABC, we shipped 3 million units outbound every day, and we must make sure that all the data is available to us on the inbound shipment. So when we send out nearly 4,000 purchase orders to manufacturers a day, we’re getting back nearly 4,000 shipments every day. Some of them are one case and some of them are multiple tractor trailer loads for a single purchase order. So it’s very important that that data for the inbound shipment to us is accurate because if it isn’t, it will impact our ability to receive the product,” said Denton.
 Jeff Denton, vice president, global secure supply chain at AmerisourceBergen Corporation (ABC).Testing delays
Jeff Denton, vice president, global secure supply chain at AmerisourceBergen Corporation (ABC).Testing delays
Hidden issues in bills impede a distributor’s ability to pick and ship the product to customers downstream. All the issues listed above lead to delays in testing. “Once an issue is identified by a system when we’re going through the test phase with a trading partner, all the processing of that file stops. So we go back and forth with manufacturers and in some cases, we’ve had to do that for three months,” he said.
This may mean emails, phone calls and possibly even conference calls. It’s a considerable amount of activity to remedy and ensure the sender understands why the file is incorrect or incomplete. “We have made zero changes in our system. It is completely designed around GS1 standards for EPCIS messaging. So what we’re doing is we’re identifying the problems, communicating back to the manufacturer and then multiple retests.”
Once the initial problem is resolved, that’s it, right? Not so fast. Once the problem is fixed and the processing passes, the system may find yet another issue, stopping the process once again. “You have to fix it and then start over. So delayed testing is a significant issue for us when we’re talking 450 trading partners and only 30 or 40 currently onboarded,” said Denton.
There isn’t enough time between now and 2023 to devote these labor-intensive remediation efforts to every trading partner. He noted, “Of course, there’s a lot of resource time on both sides with the sender and the receiver repeated retesting. And then all of this relates to increased cost.”
Help is available
The good news is that GS-1 has put together a conformance testing process, hiring service providers to perform testing of EPCIS data.
As Lerner explained, “To accomplish conformance and to take the guesswork out, GS1 US Healthcare established a GS1 Trustmark. The Trustmark signifies that an EPCIS event file follows the format and structure of the DSCSA application standard, and that this file fully conforms to a pharmaceutical traceability scenario.” Lerner’s company, Gateway Checker, established a conformance testing application which GS1 validated and certified as a GS1 conformance testing service in 2019, after a rigorous assessment against GS1 and DSCSA standards.
“This system assures that the file contains all of the appropriate information such as trading partner and drug product information, and other elements required for conforming DSCSA transactions,” said Lerner. The submitted file is then assessed against a specific drug traceability scenario. If each of the conditions are met, Gateway certifies the submission and informs GS1 that a GS1 Trustmark can be issued.
Denton added, “So you’re able to prepare your files, send it to one of these services, run it through their system very, very quickly and come back to identify” if there are issues. Manufacturers are able to resolve and retest with the service providers in a faster manner. Then when they engage with trading partners such as ABC, they know your file meets all of GS1’s standards.
“It’s a proactive measure to ensure quality in your data sharing that is extremely important, more so now than ever,” said Denton, adding that multiple service providers are available for this performance testing via GS1.
Two key points to highlight: the service providers use portals and they do not store any of the manufacturer’s data. “When you sign up with one of them, you’re able to use their portal to run your process of testing with a file that you’ve created. Test results are almost immediate, so you’re able to react.”
This means manufacturers eliminate troubleshooting emails, phone calls, and conference calls with trading partners, and they’re able to address issues and retest on their timeline.
Denton hopes that manufacturers sign up and ensure files are accurate before they come to ABC. “Hopefully we can get to a point where you process one test file and you’re accepted for being put into production. Data sharing is not easy… moving from an ASN to an EPCIS file is quite a transition. And the resources and resource skillsets are not always the same. Be proactive in the steps that are necessary for success,” he said, keeping in mind the overall goal. “The key takeaway is we must all remain responsible in our ability to deliver for healthier futures.”
Action item
Denton said to be sure you’re comfortable that the quality of your file is extremely high before testing with a trading partner. If you’re generating an EPCIS file, “I strongly recommend you go through conformance testing first.”



