hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: Technoloy
 No Comments
No Comments
3D tissue printer Prellis Biologics raises $8.7M to build replacement arteries, vascular blanks for cell cultures
3D tissue printing startup Prellis Biologics has assembled $8.7 million in new funding alongside positive news from the first transplantation of its vascular tissue scaffolds into animals.
The San Francisco-based company’s series A round was led by Khosla Ventures with additional backing from its previous seed round investors, True Ventures and SOSV’s IndieBio accelerator, to bring its total capital funding to $10.5 million.
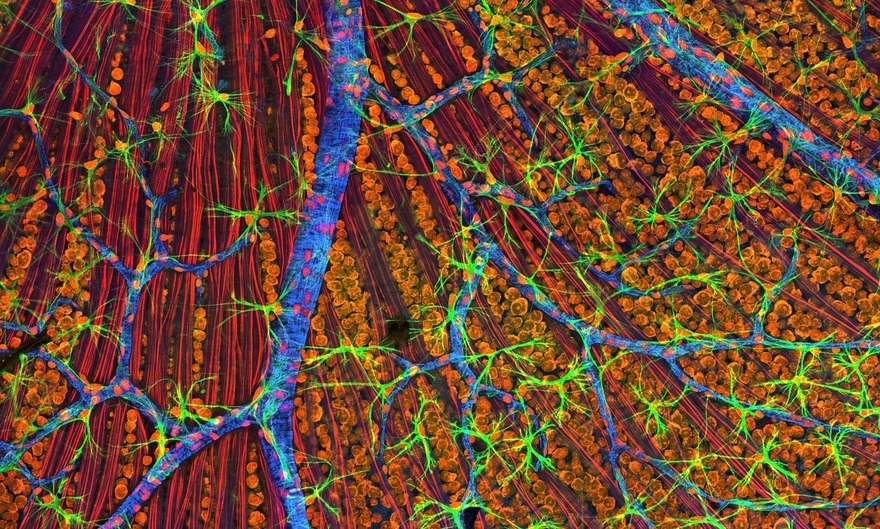
Prellis uses laser and holographic printing techniques to build 3D hydrogel structures for R&D or transplantation. This includes its line of ready-to-use biocompatible scaffolds printed with 3D capillaries, known as Vascular Tissue Blanks.
These blanks provide the underlying architecture that allows academic and pharmaceutical labs to grow their own larger tissues and organoids for study using a variety of different cell types such as neurons, stem cells, immune cells or tumor cells.
“The holy grail of human tissue engineering is the ability to build complex tissues with working vascular systems,” Prellis co-founder and CEO Melanie Matheu said in a statement. “The future of regenerative medicine revolves around harnessing the power of our own cells as therapeutics and building the tissues to keep them alive.”
In a transplantation study performed at Stanford University, human tumors built using Prellis’ vascular blanks saw full engraftment and vascularization using only 200,000 cells, according to the company.

fine capillaries to support cell growth
(Prellis)
“This is an order of magnitude fewer cells, since typical tumor studies in animals require two million or more cells,” said Matheu. “A breakthrough like this opens the door to studying rare human tumors and complex human tumor immune system reactions. It has the potential to significantly reduce overall animal use and speed up drug discovery efforts.”
The animal studies also showed the spontaneous growth of additional blood vessels into the transplanted structure over an eight-week period—including branched vasculature up to 50 microns wide, or about five times larger than a typical capillary, to connect the graft with the animal’s circulatory system.
“Spontaneous, structurally guided vascularization of our laser-printed structures is a significant milestone on the way to transplanting complex tissues,” said Matheu, adding that Prellis plans to publish the results later this year.
The company is currently testing a biomaterial interface that allows for nutrient flow and cell-to-cell interactions, the basis of the filtration and gas-exchange systems seen in the lungs and kidneys.
However, their first plans for 3D printed human organ transplantation include testing of replacement arteries about 3 mm to 4 mm in diameter in large animal studies slated for later this year.
“Arterial replacements are a natural stepping-stone to production of larger solid organs,” Matheu said. “The ones we have designed are far more sophisticated than the standard vascular replacements. They are surrounded by fine capillary beds that are known to contribute to the structural integrity and engraftment of arteries after the surgical procedure.”



