hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
VOLUNTARY RECALL OF ETHINYL ESTRADIOL & DROSPIRENONE TABLETS, USP BY JUBILANT CADISTA PHARMACEUTICALS INC. BECAUSE OF OUT-OF-SPECIFICATION DISSOLUTION TEST RESULTS
A batch of Ethinyl Estradiol & Drospirenone Tablets is recalled voluntarily by Jubilant Cadista Pharmaceuticals Inc. to the consumer level. The reason behind the recalling of the product is that there were out-of-specification dissolution results at the 3-month stability time point. Cyndea Pharma, S.L., Olvega is manufacturing the affected product is manufactured under contract from Jubilant Cadista Pharmaceuticals Inc.
Due to the OOS dissolution results, there might be a reduction in the product efficacy because of incomplete absorption of the active ingredients. Till date, Jubilant Cadista Pharmaceuticals Inc. hasn’t received any reports of adversative events related to this recall.
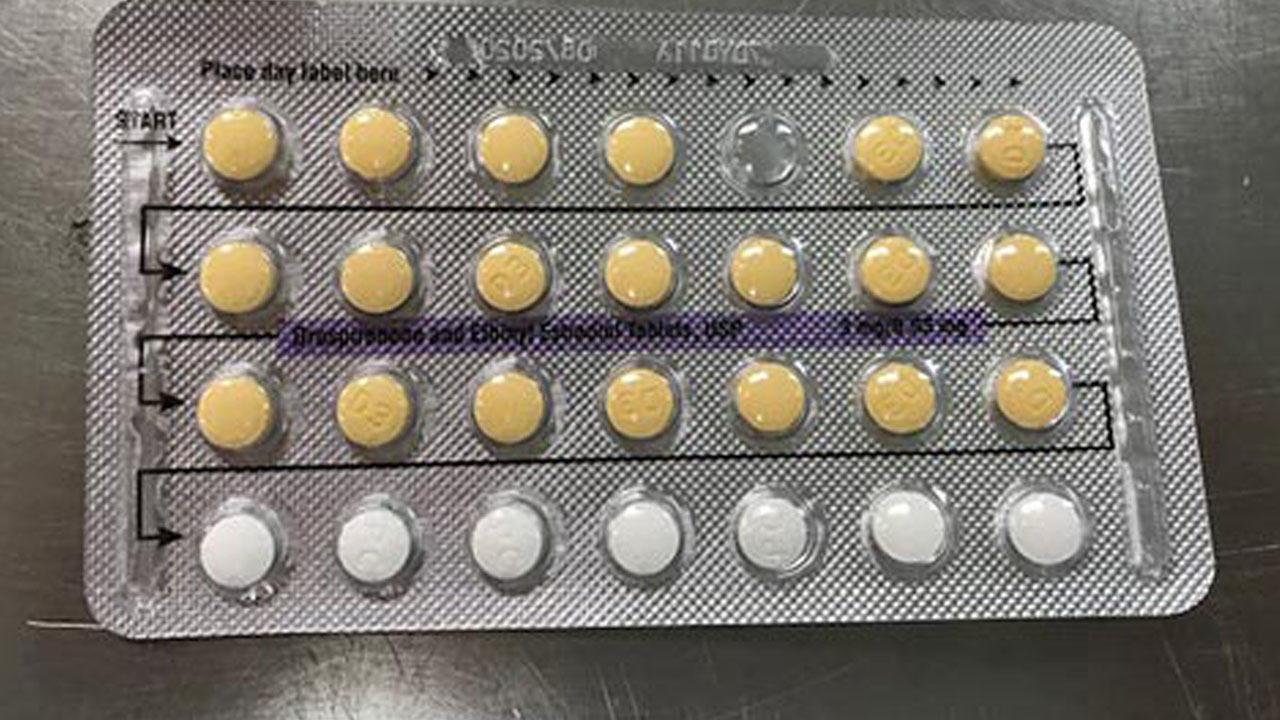
Product Description: Ethinyl Estradiol and Drospirenone tablets are a progestin/estrogen combination oral contraceptive, specified for use by women to prevent pregnancy. Treat symptoms of the premenstrual dysphoric disorder for women who prefer to use an oral contraceptive for contraception.
Treat mild level of acne for women aging at least 14 years old or/and if the patient desires an oral contraceptive for birth control
Ethinyl Estradiol and Drospirenone tablets are packed into a carton enclosing 3 blister cards. Each blister card is inclusive of biconvex tablets, 28-film-coated.
The affected Ethinyl Estradiol and Drospirenone Tablets, USP, has been recognized as Lot number 183222, with NDC number 5974676343 and expiry date of November 2020.
Ethinyl Estradiol and Drospirenone Tablets were distributed all across the nation to wholesalers, distributors, & retailers.
Jubilant Cadista Pharmaceuticals Inc. is giving notifications to its customers by sending them an email of a recall notification letter & response form. Also, they are arranging for return of all recalled product.
Patients who already used the affected lot Ethinyl Estradiol and Drospirenone tablets, USP, 3 mg/0.02 mg should consult their healthcare provider as soon as possible. Patients may also return the affected lot to their place of purchase.



