hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
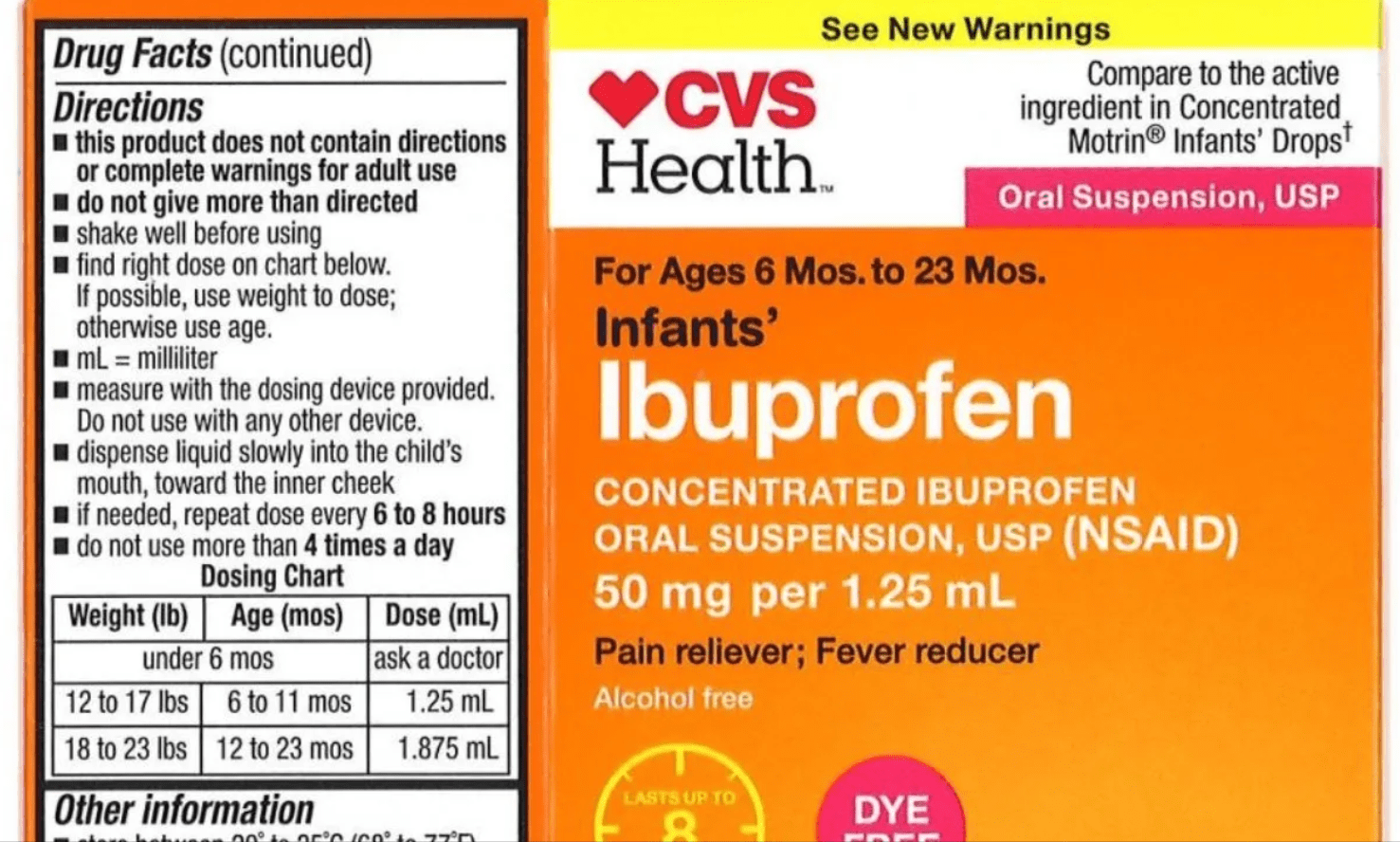
CVS, Walmart expand infant ibuprofen recall
According to the USDA, some CVS Health and Equate brand liquid infants’ ibuprofen may have higher concentration levels of ibuprofen than the label states.
“Infants already susceptible to the adverse effects of ibuprofen may be at a slightly higher risk if they receive medication from an impacted bottle. There is a remote probability that infants, who may be more susceptible to a higher potency level of drug, may be more vulnerable to permanent NSAID-associated renal injury,” the USDA said.
The bottles in question are marked “Concentrated Ibuprofen Oral Suspension, USP” and have a concentration of 50 mg of ibuprofen per 1.25 mL. They are sold in 0.5 ounces and 1-ounce packages.
Most children can handle about 700 percent of the recommended dosages of ibuprofen and no serious injuries have been reported.
The products were distributed to Walmart and CVS. A previous recall from the same parent company, Tris Pharma, impacted 0.5 bottles sold under Family Dollar’s Family Wellness brand.
Here are the full details on the recalled products:
– CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 0.5 oz. bottle, expiration date 12/19, sold at CVS Pharmacy
– Equate: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle, expiration date 2/19, sold at Walmart
– CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle, expiration date 2/19, sold at CVS Pharmacy.
Source: https://www.rfdtv.com/story/41508806/cvs-walmart-expand-infant-ibuprofen-recall



