hidden
Over 10 years experience of Traceability Solutions
By Pharmatrax Author
Category: News
 No Comments
No Comments
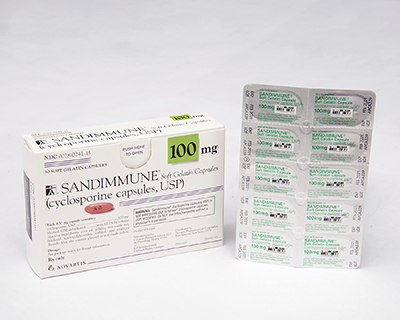
Novartis recalls immunosuppressant Sandimmune, Neoral capsules for hazardous packaging
Novartis, like many generic drugmakers, was forced to pull copies of generic Zantac off shelves late last year after a carcinogen scare shocked the industry. Now, it’s once again pulling products off shelves—and this time, it’s two branded drugs whose packaging has proved hazardous to children.
Novartis will recall U.S. lots of 100-milligram Sandimmune and Neoral, immunosuppressant capsules used to treat organ rejection after a transplant, due to blister packs that were found not to be child-resistant, the drugmaker said.
The 25-milligram doses of both drugs are not part of the Consumer Product Safety Commission-backed recall and will remain on shelves, Novartis said. The drugmaker noted the recall was not tied to the drugs’ efficacy and told patients to continue taking the med at the direction of their physicians.



