Over 10 years experience of Traceability Solutions

The British Heart Foundation’s “Big Beat Challenge” has revealed its four finalists, which will now compete to win a £30 million funding award. The organization…
CONTINUE READINGDuring Q2 FY2020, India witnessed the outbreak of many diseases in many parts, aiding the growth of the anti-infective segment, he added. The growth trajectory…
CONTINUE READING

Back in December 2018, with Johnson & Johnson reeling from a bombshell Reuters article on talc, CEO Alex Gorsky appeared in a video to reassure…
CONTINUE READINGThis past September the U.S. Food and Drug Administration issued a statement warning that “the FDA has learned that some ranitidine medicines, including some products…
CONTINUE READING

According to the FDA, ABH NATURE’S PRODUCTS, INC, ABH PHARMA, INC., and STOCKNUTRA.COM, INC. (the “COMPANIES”) is recalling all lots of its dietary supplements sold nationwide because…
CONTINUE READINGMylan Recalls 3 Lots of Nizatidine The recall includes 150 mg and 300 mg dosages of the capsules, which are used to treat the following:…
CONTINUE READING

You’re unwell, you see a doctor, they prescribe you a medicine… and you take it. But how exactly is that drug having an effect? What is its…
CONTINUE READINGThe FDA has added at least three additional items to its growing roster of heartburn drug recalls. The onslaught of new recalls were announced between…
CONTINUE READING
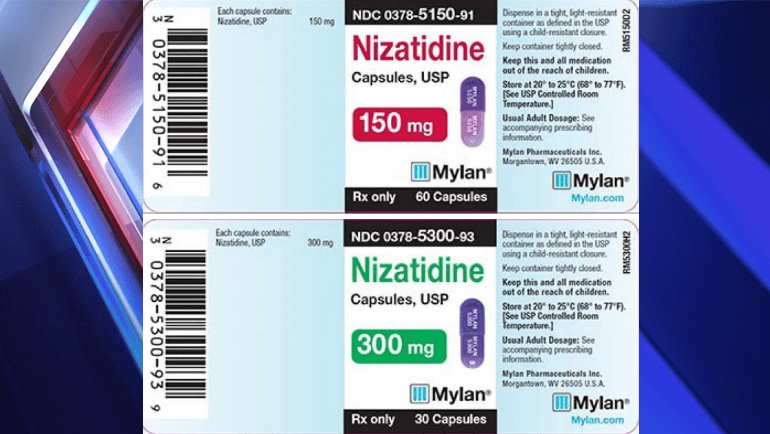
WASHINGTON D.C. — Mylan Pharmaceuticals is conducting a voluntary recall of three lots of Nizatidine Capsules, according to the U.S. Food & Drug Administration (FDA).…
CONTINUE READINGThe company is making the agency’s open-source MyStudies platform available, enabling for easier access to data for research into new medications, devices and more. Google…
CONTINUE READING