Over 10 years experience of Traceability Solutions

A study has suggested that while funding for artificial intelligence in pharma was $5.2bn in 2019, overall investment is slowing. New research has revealed that…
CONTINUE READINGMylan Pharmaceuticals has issued a voluntary nationwide recall of three lots of Nizatidine Capsules due to a substance that could cause cancer. Three lots of…
CONTINUE READING

(WAFB) – Particular depression medication is being recalled due to a labeling error related to the dosage strength of the medicine. Aurobindo Pharma USA, Inc.…
CONTINUE READINGAccording to the USDA, some CVS Health and Equate brand liquid infants’ ibuprofen may have higher concentration levels of ibuprofen than the label states. “Infants…
CONTINUE READING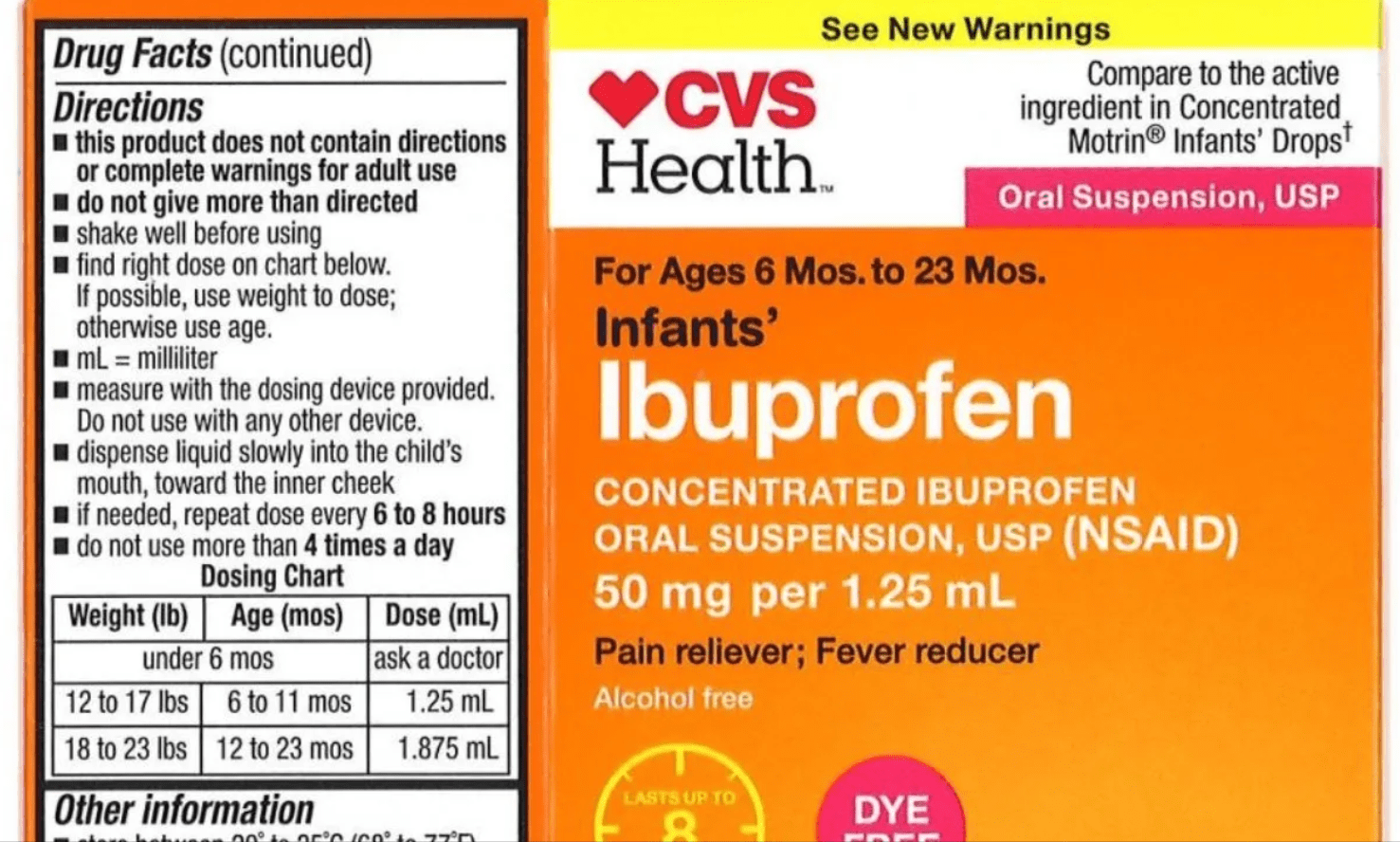
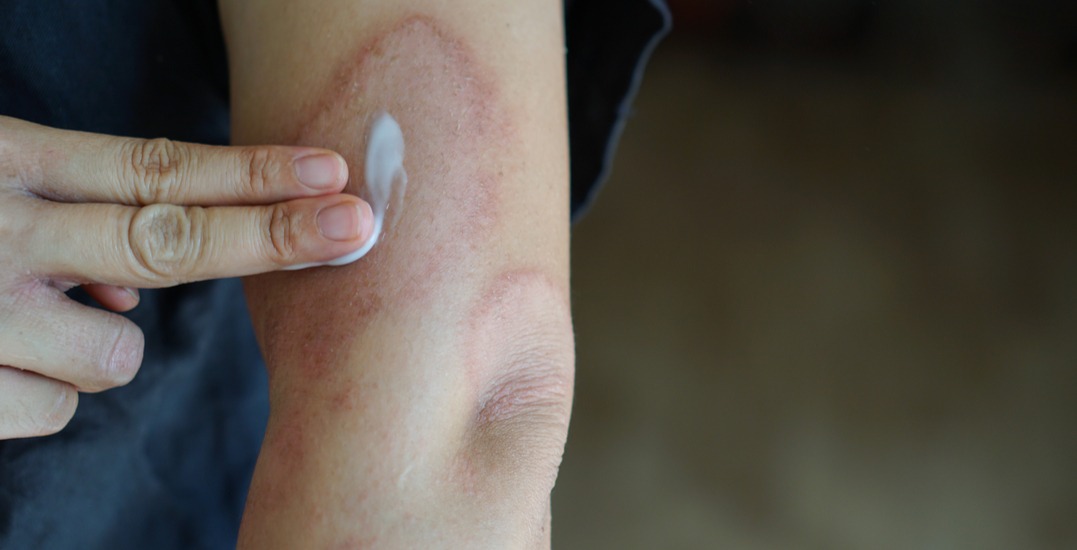
Taro Pharmaceuticals Inc. is recalling one lot of Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream, due to a labelling error that may pose serious health risks…
CONTINUE READINGMONMOUTH JUNCTION, N.J. – Editor’s Note: There was no new expansion of the recall in December 2019, as a previous version of this story suggested. The original…
CONTINUE READING
(HealthDay)—Recall of a cardiac resynchronization therapy pacemaker, which occurred in November 2015, was delayed unnecessarily, according to a report published online Dec. 20 in JAMA Internal…
CONTINUE READINGEntrepreneurs and executives are using the technology to create data markets and to reduce the burden of bureaucracy. The hype around blockchain in healthcare has…
CONTINUE READING
The tablets are being recalled because of the presence or potential presence of N-nitrosodimethylamine (NDMA) levels above the acceptable daily intake levels established by the…
CONTINUE READINGWhen the media surfaces in product recall, they also give rise to customer fears and frustration. What is the essence of a reminder? Why is…
CONTINUE READING