Over 10 years experience of Traceability Solutions
WASHINGTON, DC (WICS/WRSP) — A blood pressure medicine that has dealt with at least four recalls this year is facing yet another. This time, the recall…
CONTINUE READINGComputer model predicts worsening cognitive scores, opening door to the use of machine learning in the selection of participants in Alzheimer’s disease trials. One of…
CONTINUE READING

The FDA posted a public missive alongside a warning letter to a Chinese OTC drug manufacturer, highlighting the efforts it had been making to address…
CONTINUE READINGMedAdvisor, a digital medication management company, has announced it will launch in the UK market in early 2020, having secured its first UK pharmacy chain…
CONTINUE READING
Artificial Intelligence can predict acute kidney injury (AKI) 48 hours before it happens, according to new research. Google’s DeepMind can predict this leading cause of…
CONTINUE READINGJubilant voluntarily recalls one lot of contraceptive tablets due to out-of-specification dissolution results, potentially decreasing efficacy. Jubilant Cadista Pharmaceuticals, a generics pharmaceutical company, announced the…
CONTINUE READING
A batch of Ethinyl Estradiol & Drospirenone Tablets is recalled voluntarily by Jubilant Cadista Pharmaceuticals Inc. to the consumer level. The reason behind the recalling…
CONTINUE READINGA batch of popular painkillers has been pulled from pharmacy shelves after another medication that is four times stronger was found in one packet. The…
CONTINUE READING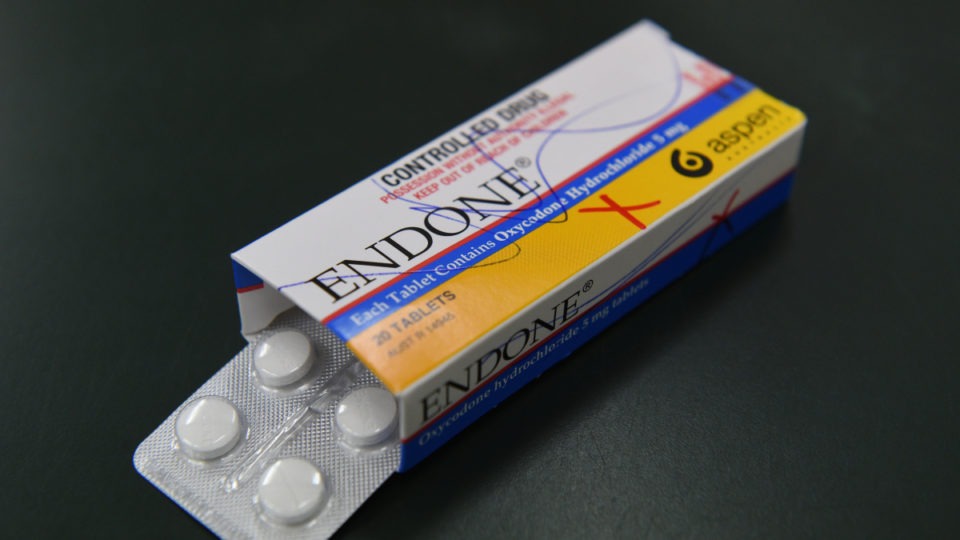
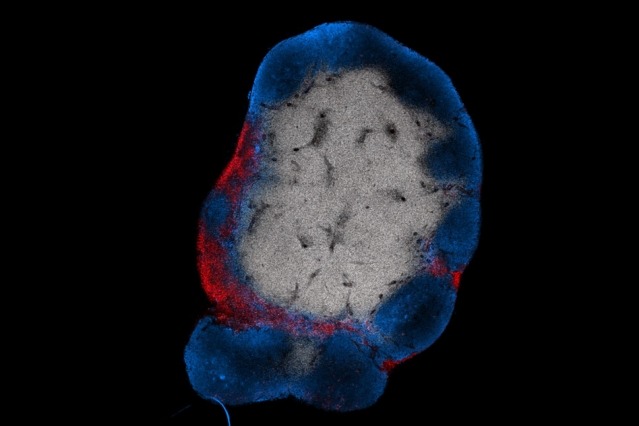
Super-charging a treatment for leukemia also makes it effective on solid tumors. A promising new way to treat some types of cancer is to program…
CONTINUE READINGChina and India are both major API suppliers and generic-heavy countries. Because of the similarity, Indian drugmakers have long had a hard time finding an…
CONTINUE READING