Over 10 years experience of Traceability Solutions
The manufacturer company, Tris Pharma recall infant ibuprofen sold by several national retailers. Additionally, a recall has been issued for the medicine sold by CVS,…
CONTINUE READINGThe time could be coming when you can order your medicine online and have it delivered to your home. In the US, online shopping giant…
CONTINUE READING

Dive Brief: The Food and Drug Administration issued its annual report Monday on the state of pharmaceutical quality, highlighting its inspection efforts and offering geographic and industry…
CONTINUE READINGSix years after federal prosecutors first targeted Novartis for an alleged kickback scheme involving $10,000 dinners and fishing trips for doctors, signs pointed to the…
CONTINUE READING
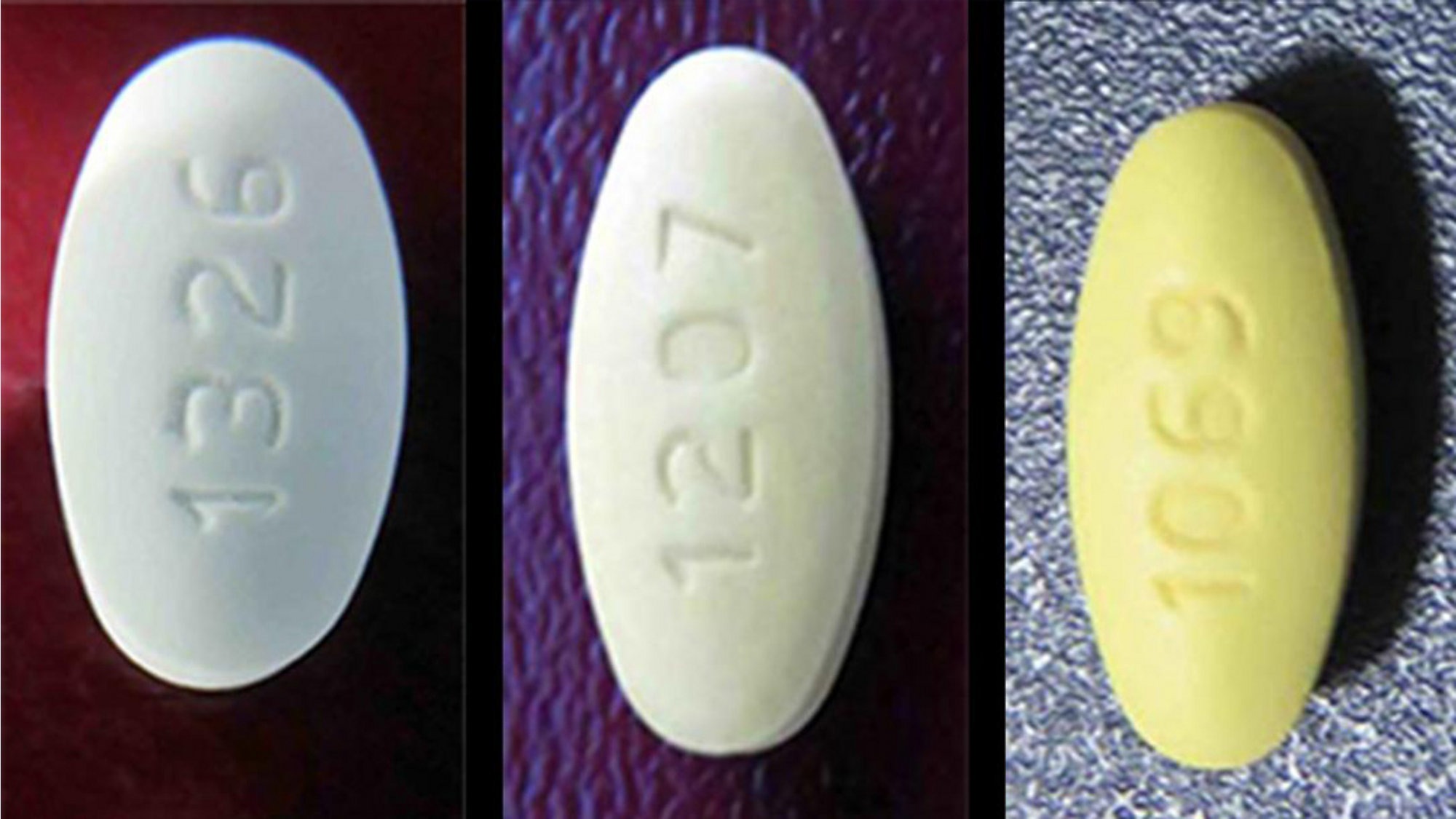
A year after Torrent Pharmaceuticals first began recalling tainted losartan products manufactured at its Indrad plant, the FDA paid a visit. It found a plant…
CONTINUE READINGPar Pharmaceutical initiates a recall of mycophenolate mofetil injection, after a vial containing a glass fragment was discovered during reconstitution. The voluntary recall involves only…
CONTINUE READING
Even as more felony charges may follow in drug epidemic, sleep med warning suggests pill popping stays too popular Five top executives at a major…
CONTINUE READINGTeva Pharmaceuticals, on Friday, announced it is recalling its blood pressure medication, Losartan Potassium USP. All 35 lots of the drug, which includes 100 mg…
CONTINUE READING

Zydus Pharmaceuticals USA Inc, a subsidiary of Zydus Cadila, is also recalling 7,668 bottles of Carvedilol Tablets, used to treat high blood pressure, in the…
CONTINUE READINGWhile Dr Reddy’s Laboratories initiated recall after their drugs were found to be exposed to above 50 per cent relative humidity levels during packaging operations,…
CONTINUE READING