hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
Coronavirus Treatment Could Lie in Existing Drugs
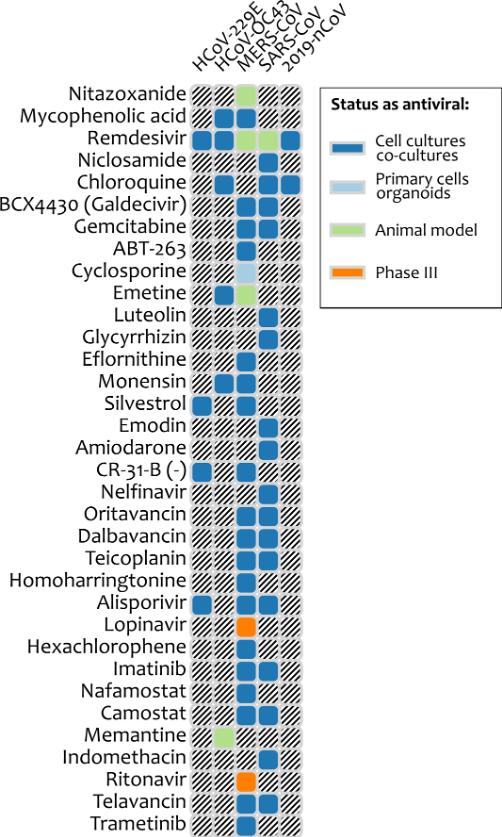
As the global number of COVID-19 cases passes 81,000, collaborating European scientists have identified 31 existing broad-spectrum antiviral agents (BSAAs) that they say may represent candidates for repurposing against the infection. The researchers suggest that repositioning existing approved and investigational drugs may represent the key to future fights against viral infections — including the SARS-CoV-2 virus, and other emerging viruses — and they have compiled a database that summarizes the activity and development status of more than 100 safe-in-man BSAAs.
“Drug repurposing is a strategy for generating additional value from an existing drug by targeting diseases other than that for which it was originally intended,” said Denis Kainov, Ph.D., senior author on the paper and an associate professor at the Norwegian University of Science and Technology (NTNU). “For example, teicoplanin, oritavancin, dalbavancin, and monensin are approved antibiotics that have been shown to inhibit corona- and other viruses in the laboratory.” Kainov and his co-authors say that these and other already tested safe-in-man, broad-spectrum antiviral agents (BSAAs) are good starting candidates.
The researchers are making their database freely available, and report on their findings in the International Journal of Infectious Diseases, in a paper titled “Discovery and development of safe-in-man broad-spectrum antiviral agents.” Their report coincides with the start of U.S. trials with Gilead Sciences’ antiviral drug, remdesivir, which was previously tested as a treatment for the Ebola virus.
BSAAs are small-molecules that may inhibit different types of human viruses that exploit similar pathways and host factors to replicate inside cells, the authors explained. The advantage of repurposing a drug is that all of the details surrounding the drug development are already known, from the chemical synthesis steps and manufacturing processes to information regarding the different phases of clinical testing.
“Although the concept of BSAAs has been around for almost 50 years, the field received a new impetus with recent outbreaks of Ebola, Zika, Dengue, influenza and other viral infections, the discovery of novel host-directed agents as well as the development of drug repositioning methodology,” the investigators noted. “Therefore, repositioning of launched or even failed drugs to viral diseases provides unique translational opportunities, including a substantially higher probability of success to market as compared with developing new virus-specific drugs and vaccines, and a significantly reduced cost and timeline to clinical availability.”
SBAAs could thus hold promise for treating the infection with the SARS-CoV-2 virus. “No vaccines and drugs are available for prevention and treatment of coronavirus infections in humans,” they stated. “However, safe-in-man BSAAs could be effective against 2019-nCoV [SARS-CoV-2 virus] and other coronaviruses.” For example, they pointed out, chloroquine and remdesivir effectively inhibited infection by the SARS-CoV-2 virus in vitro. “Moreover, teicoplanin, oritavancin, dalbavancin, monensin, and emetine could be repurposed for treatment of 2019-nCoV infections,” the team noted. “Oritavancin, dalbavancin, and monensin are approved antibiotics, whereas emetine is an anti-protozoal drug. These drugs have been shown to inhibit several corona- as well as some other viral infections.”
The researchers reviewed information on the discovery and development of BSAAs and summarized what they found for 120 approved, investigational and experimental drugs that had already been shown to be safe for humans use, and which inhibit 86 human viruses in 25 viral families. “The BSAAs inhibit viral or host factors and block viral replication, reduce the viral burden to a level at which host immune responses can deal with it or facilitate apoptosis of infected cells,” the authors noted. They suggested that 31 of the SBAAs could represent possible candidates for prophylaxis and treatment of infection by SARS-CoV-2.
The team has compiled their findings into a BSAA database, which is freely available at Drugvirus.info. “The database allows interactive exploration of virus-BSAA interactions,” they stated. “It also includes information on BSA targets.”
The investigators also highlight the potential to combine drugs against viruses. “By contrast to individual drugs, combinations of 2-3 BSAAs could be used to target an even broader range of viruses,” they noted. “Such combinations could serve as front line therapeutics against poorly characterized emerging viruses or re-emerging drug-resistant viral strains.” Kainov and colleagues further suggest that making the results of relevant clinical trials publicly available and standardizing data collection will aid the prioritization and translation of emerging and existing BSAAs into clinical practice. “This would allow BSAAs to play a pivotal role in the battle against emerging and re-emerging viral diseases.”
Source: https://www.genengnews.com/news/coronavirus-treatment-could-lie-in-existing-drugs/



