hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
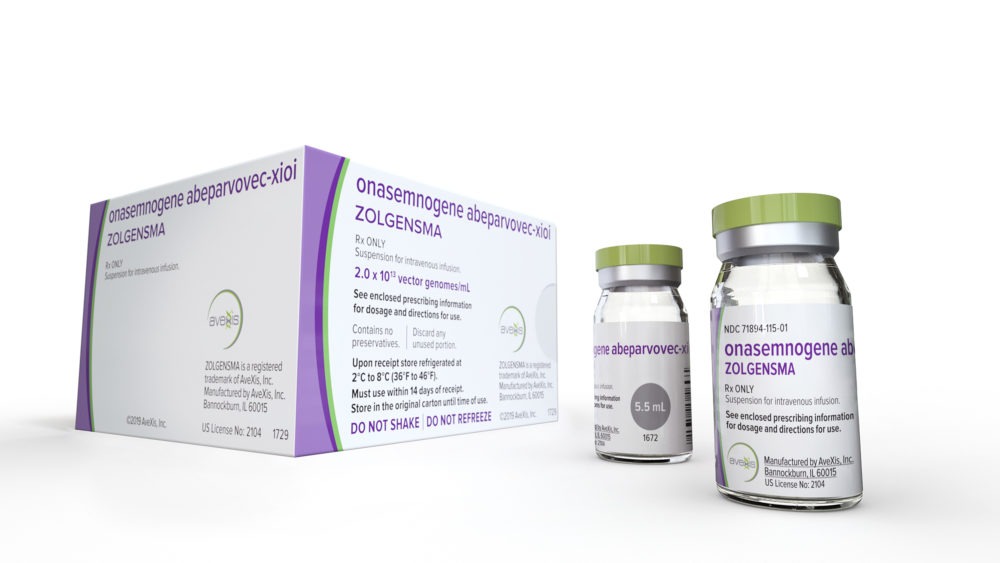
FDA Approves Drug With Record-Breaking List Price: $2.1 Million
The FDA on Friday approved a one-time gene therapy that treats a rare genetic disease — for a record-breaking sticker price of $2.1 million.
The approval of Zolgensma, made by Novartis, adds a new layer to a debate raging in the United States and other countries: What is a fair price for a treatment that can save something priceless, a human life?
It’s a question patients and policymakers are debating ever more earnestly as pharmaceutical companies invent ways to combat debilitating conditions, but at exorbitant costs.
Zolgensma treats spinal muscular atrophy (SMA), which attacks nerve cells that control basic movements, like breathing and swallowing. SMA is the leading genetic cause of death for infants.
On a conference call with reporters and stock analysts Friday, Novartis CEO Vas Narasimhan predicted most patients won’t pay full price.
“We expect the patient out-of-pocket [cost] to be limited, and we have patient-support programs to ensure that patients have broad access,” he said.
In a boost for Novartis, the Institute for Clinical and Economic Review, said Zolgensma’s list price is reasonable. Boston-based ICER is an influential drug-cost watchdog.
“The price that was announced today seems extraordinary, but this is an extraordinary treatment,” ICER President Steven Pearson said in an interview. “It’s a one-time treatment that will essentially cure — according to the short-term data — a fatal disease, the leading genetic cause of disease in infants. If we consider that significant clinical benefit, this price aligns fairly.”
Zolgensma is now a competitor to Spinraza, a drug made by Cambridge-based Biogen. An ongoing therapy, Spinraza bears a list price of $750,000 in the first year and $375,000 per year after that; with insurance, however, many patients pay far less, out of pocket.
Source: https://www.wbur.org/commonhealth/2019/05/24/zolgensma-most-expensive-gene-therapy



