hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
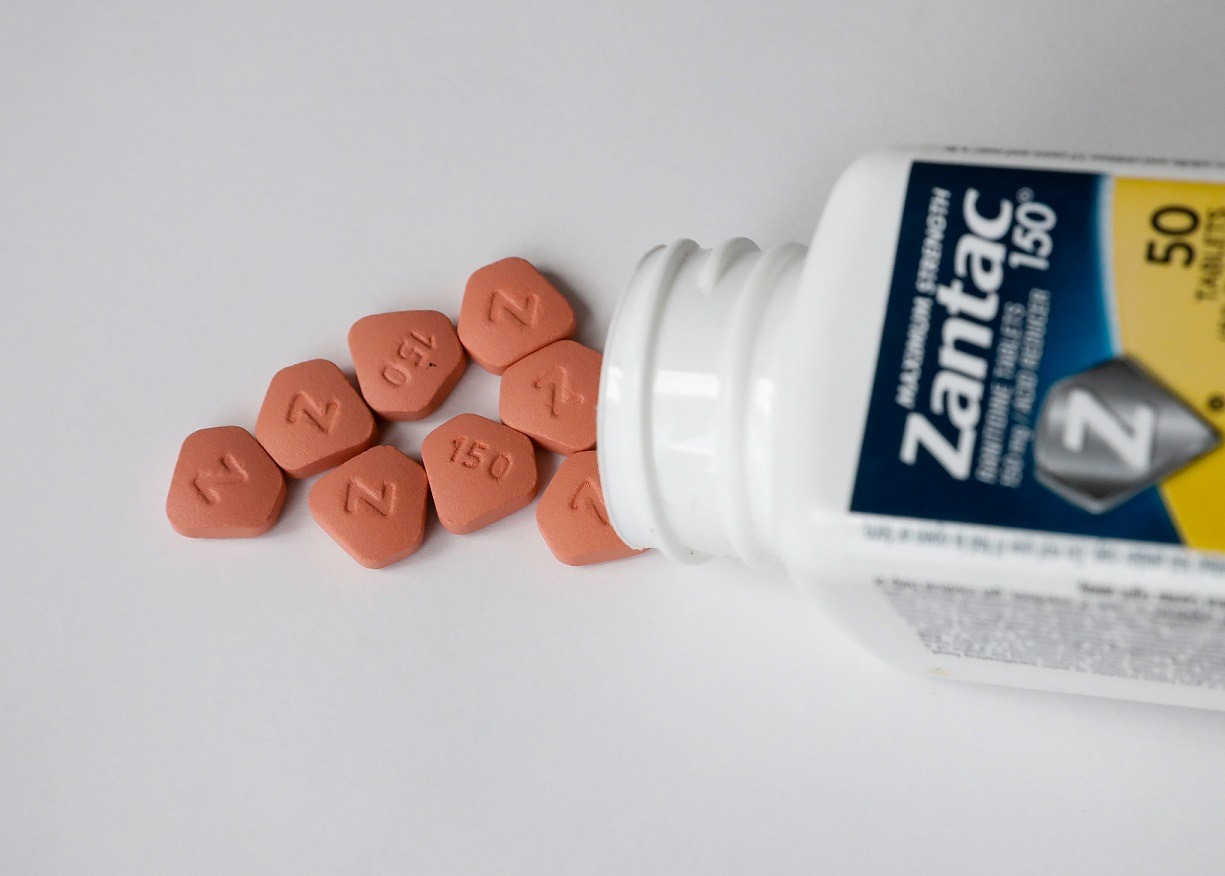
Four more manufacturers recall ranitidine products
OTC Concepts Ltd, Relconchem Ltd, Noumed Life Sciences Ltd, and Medreich Plc have recalled all unexpired batches of certain ranitidine 75mg tablets from pharmacies and retail stores.
The latest alert, revealed by PSNC, comes a day after Creo Pharma and Tillomed Laboratories recalled ranitidine medicines.
The recall is a precautionary measure due to the possible contamination with impurity N-nitrosodimethylamine (NDMA).
Pharmacists are asked to stop supplying the recalled products immediately and remove them from the store shelves. The stocks should be returned to the supplier through their approved process.
In October, GlaxoSmithKline (GSK) and Teva UK have recalled their ranitidine products soon after the US, Swiss and German regulators found “unacceptable” levels of NDMA, which has genotoxic and carcinogenic potential, in the popular heartburn drug.
Omega Pharma Limited, Galpharm International and Rosemont Pharmaceuticals have also recalled their ranitidine products.
Product details:
OTC Concepts Ltd
| Product | PL Number |
| Ranitidine 75mg Tablets | 20338/0020 |
Relonchem Limited
| Product | PL Number |
| Relonchem Ranitidine 75mg Tablets (6) | 20395/0079 |
| Relonchem Ranitidine 75mg Tablets (12) | 20395/0079 |
| Bells Healthcare Ranitidine 75mg Tablets (12) | 20395/0079 |
| Numark Ranitidine 75mg Film-Coated Tablets (12) | 20395/0079 |
| Tesco Heartburn and Indigestion Relief 75mg Film-Coated Tablets (12) | 20395/0079 |
Noumed Life Sciences Ltd
| Product | PL Number |
| Noumed Indigestion Relief 75mg Film-Coated Tablets | 44041/0027 |
| Gavilast Heartburn and Indigestion 75mg FilmCoated Tablets | 44041/0028 |
| Ranitidine 75mg Film-Coated Tablets | 44041/0028 |
Medreich Plc
| ASDA Ranitidine 75 mg Tablets (12) | PL 21880/0022 | ||
| Batch Number | Expiry Date | Pack Size | First Distributed |
| 370190 | 31/01/2020 | 1 x 12 | August 2017 |
| Peach Ethical Indigestion Relief Ranitidine 75mg Film-Coated Tablets (12) |
PL 21880/0022 | ||
| Batch Number | Expiry Date | Pack Size | First Distributed |
| 370049 | 31/12/2019 | 1 x 12 | October 2017 |
| Sainsbury’s Indigestion Relief Tablets (12) | PL 21880/0022 | ||
| Batch Number | Expiry Date | Pack Size | First Distributed |
| 370065 | 31/12/2019 | 1 x 12 | May 2017 |
| 370066 | 31/12/2019 | 1 x 12 | May 2017 |
| Tesco Indigestion Relief Ranitidine 75mg film-coated tablets |
PL 21880/0022 | ||
| Batch Number | Expiry Date | Pack Size | First Distributed |
| 370050 | 31/12/2019 | 1 x 12 | March 2017 |
| 370051 | 31/12/2019 | 1 x 12 | March 2017 |
| 370052 | 31/12/2019 | 1 x 12 | April 2017 |
| 370064 | 31/12/2019 | 1 x 12 | May 2017 |



