hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
RECALL: Mylan Pharmaceuticals says trace elements of NDMA found in some antacids
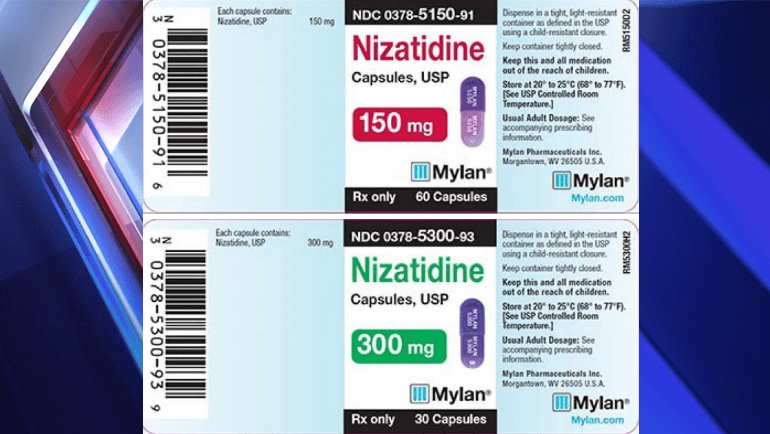
WASHINGTON D.C. — Mylan Pharmaceuticals is conducting a voluntary recall of three lots of Nizatidine Capsules, according to the U.S. Food & Drug Administration (FDA).
The FDA website said three lots of Nizatidine capsules are being voluntarily recalled due to detected trace amounts of an impurity N-nitrosodimethylamine (NDMA) contained in the API Nizatidine, USP, manufactured by Solara Active Pharma Sciences Ltd.
A known environmental contaminant found in water, and foods like meats, dairy products and vegetables, NDMA is classified as a probable human carcinogen, according to the International Agency for Research on Cancer (IARC).
FDA said Nizatidine is used to treat ulcers, gastroesophageal reflux disease and conditions that cause too much stomach acid.
According to FDA, Mylan has not received any reports of adverse events related to these batches.
These batches manufactured by Mylan Pharmaceuticals were distributed nationwide to wholesalers, mail order pharmacies, retail pharmacies, and a distributor between June 2017 and August 2018. The recalled batches are as follows:
0378-5150-91, Nizatidine Capsules USP, 150mg, Bottles of 60, Lot #3086746, Exp. May 2020
0378-5300-93, Nizatidine Capsules USP, 300mg, Bottles of 30, Lot #3082876, Exp. Jan 2020
0378-5300-93, Nizatidine Capsules USP, 300mg, Bottles of 30, Lot #3082877, Exp. Jan 2020
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.



