hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: Technoloy
 No Comments
No Comments
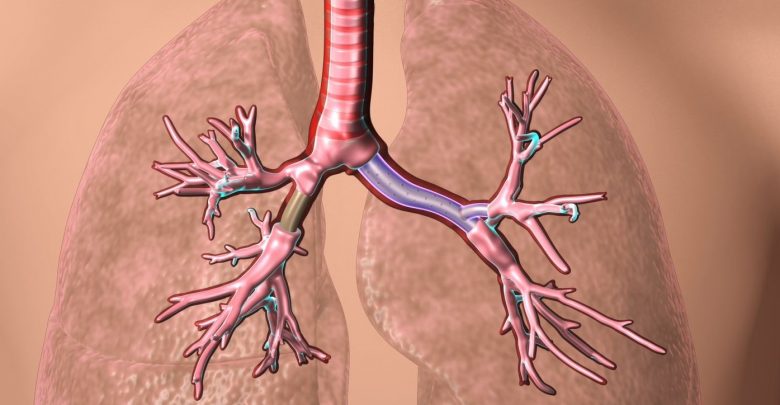
3D printed patient-specific airway stents approved by FDA
The U.S. Food and Drug Administration (FDA) has issued approval for patient-specific airway stents made with 3D printing. The custom stents, which are used to help patients with breathing disorders, were developed by Dr. Tom Gildea, a physician at the Cleveland Clinic.
The recently approved stents can now be widely implemented to help patients suffering from serious breathing disorders caused by trauma, tumors, inflammation, etc. The customizable stents, which are made using a combination of 3D printing and silicone injection molding, have been used in the past but in a limited capacity. That is, the patient-specific breathing devices were viable under the FDA’s compassionate use program, which enables patients to seek out investigational treatment solutions if all available treatments have failed.
The stents developed by Dr. Gildea and his team are based on data from CT scans, which is transformed into a 3D printable mold using a proprietary 3D visualization software. The stent molds are subsequently 3D printed and injected with medical-grade silicone. The resulting device is a stent whose shape and size are perfectly matched to the patient’s anatomy.
Traditional airway stents, available in a limited range of sizes, do not always fit the patient, which can lead to airway complications and discomfort. An ill-fitting stent can cause a number of problems, including stent kicking and bending, as well as new tissue growth, tissue death or mucus impaction.

The patient-specific stents, however, can be tailored to the patient, resulting in easier implantation and overall less risk. Moreover, the 3D printed stents are reportedly more long-lasting than traditional stents. According to studies, the customized stents only had to be replaced after a year—four times the lifespan of 90-day conventional stents. The patient-specific silicone stents have also been shown to result in shorter procedure times and improved patient-reported symptoms.
“Breathing is something many people take for granted, but for many of these patients, every breath can be a struggle. It’s been gratifying to see patients receiving the customized stents feeling relief right away.” said Dr. Gildea, section head of bronchoscopy at Cleveland Clinic. “We are excited to be able to bring this technology to more patients across the country and grateful for the patients and donors who have worked with us to help pioneer this technology.”
The team at the Cleveland Clinic responsible for developing the 3D printed stent will also form a new subsidiary called VisionAir Solutions to promote and deliver personalized medical devices to interventional pulmonologists and their patients. Within the first quarter of 2020, the new company expects to deploy the patient-specific stents to patients “in a controlled launch” across many of the U.S.’ top medical institutions.
3D printed patient-specific stents are gaining traction in the medical sector. In October, for instance, Italian company Prosilas 3D printed a stent which was successfully implanted into a five-year-old boy with tracheomalacia. Dr. Glenn Green from the University of Michigan has also been recognized as a pioneer in the niche sector, developing a 3D printed splint to treat tracheobronchomalacia in young children in 2015.
Source:https://www.3dprintingmedia.network/3d-printed-airway-stents-fda-approved/



