hidden
Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
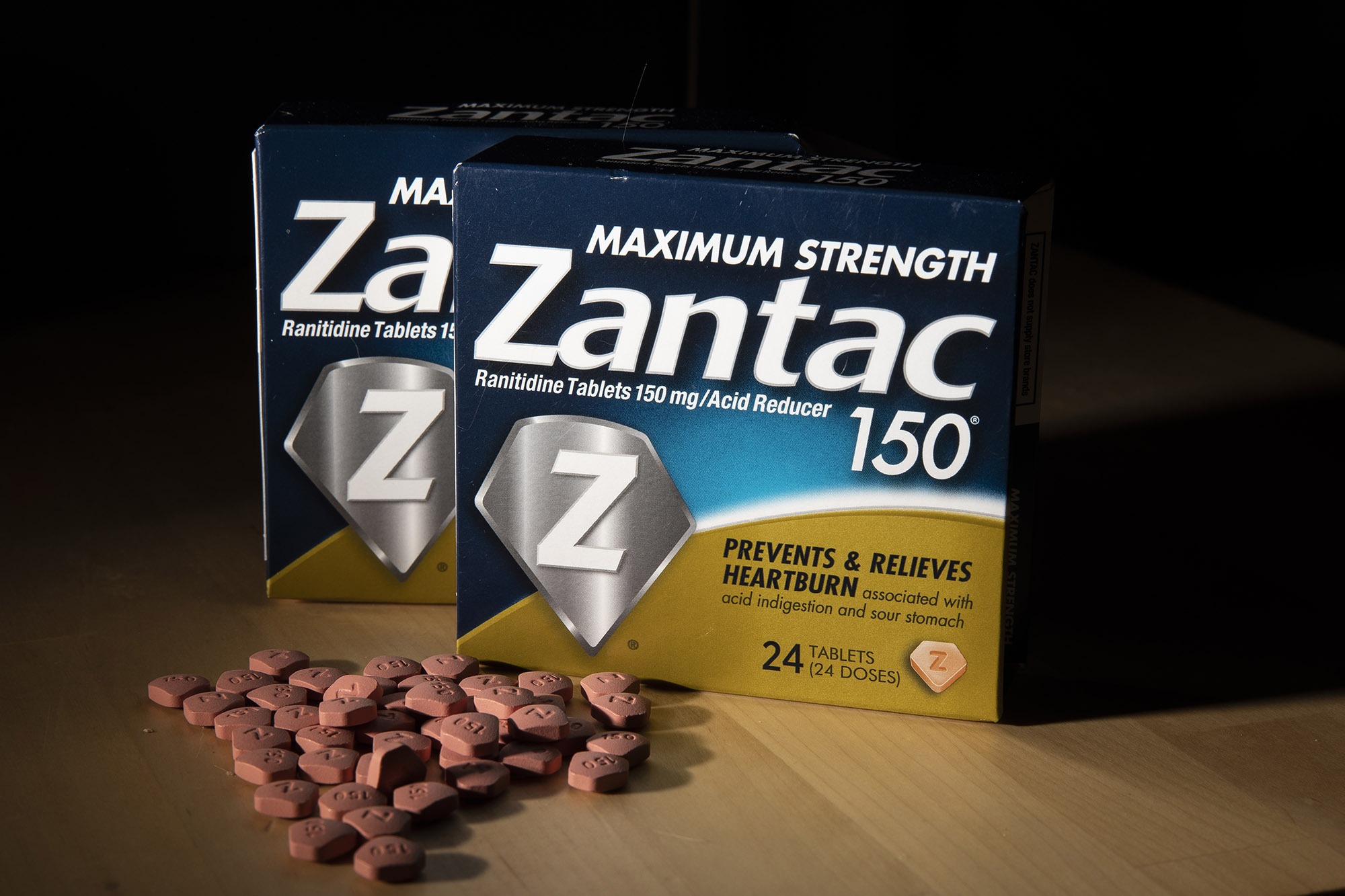
Zantac To Be Recalled in U.S. and Canada on Carcinogen Concern
Sanofi is voluntarily recalling heartburn medication Zantac in the U.S. and Canada amid worries that the medicine may be tainted with a compound that could cause cancer.
The French pharma company’s announcement follows decisions from major drugstore operators in the U.S., including Walgreens Boots Alliance Inc. and Walmart Inc., to pull the medication from store shelves. In addition, manufacturers have recalled some generic versions of Zantac products known as ranitidine.
Due to inconsistencies in preliminary test results of the key ingredient used in the U.S. and Canadian products, Sanofi decided to carry out the recall in those countries as an investigation continues. The company said it’s working with health authorities to determine the level and extent of the recall.
U.S. and European Union health officials are investigating levels of the probable carcinogen NDMA in Zantac and its generic equivalent sold by numerous other companies. The FDA has advised patients that there are other medications available to treat the same symptoms that ranitidine is intended to soothe.
Branded Zantac is made by Sanofi, while generic versions are manufactured by many companies. Perrigo Co. earlier this month was the latest to halt distribution of the generic version. Novartis AG’s generics unit Sandoz and Dr. Reddy’s Laboratories Ltd. of India had already taken similar steps.
Sanofi sources the active ingredient for the over-the-counter product from Spain, according to the company.
Active ingredients used in Sanofi’s ranitidine products outside the U.S. and Canada come from different suppliers, the company said. Sanofi won approval beginning in 1995 to sell branded Zantac without a prescription. The product had sales of 69 million euros ($77 million) in the first half of the year, a tiny fraction of Sanofi’s roughly 17 billion euros in total revenue for the period.



